Speaking of company compliance, ethics and vision
July 5, 2023
Mondeo is investing in growth
October 19, 2023
- Passivation determines stainless steel resistance
- Electropolishing amplifies the passivation level of stainless steel
- The polarisation curve test measures the level of passivation
Spontaneous passivation and stainless steel passivation treatment
When talking about AISI 304, even more if AISI 316 stainless steel, you can be sure that you are discussing a material which is extremely corrosion resistant.
What makes stainless steel corrosion resistant is its passivation, namely the formation of an invisible protective film made up mainly of chrome oxides and hydroxides, which protect the rest of the metal from further oxidation.
Spontaneous passivation is an unprompted process coming from the activity of oxygen on the surface of the metal. Induced passivation, which accelerates and amplifies the process to promote the formation of chrome oxide, is a process that stands alongside it.
Thanks to an advanced electropolishing system, all our stainless steel valves and manifolds are electropolished, making them much more resistant.
Electropolishing is the most effective system for increasing passivation: in this case the metal acts as the anode in an electrolytic cell and passivation is electrochemical. The shine that the metal acquires when treated, the result of the exposed chrome on the surface, is in itself a sign of greater passivation.
Electropolishing increases the passivation level of stainless steel
We have already seen how the salt spray test demonstrates the effectiveness of electropolishing in increasing resistance to corrosion. In this case, the presence or absence of signs of corrosion clearly show the results of the test.
Here, instead, we want to present the results of additional research based on polarisation curves, which allowed us to assess numerically the increase in passivation after electropolishing. To confirm the suitability of our electropolishing process in rendering stainless steel products more stainless, we commissioned the test to sector experts, who did the corrosion tests in the electrochemical laboratories of the DIEF (‘Enzo Ferrari’ Department of Engineering, University of Modena and Reggio Emilia).
The results have shown the efficiency of electropolishing treatment
Five test samples were used: one of AISI 304 that had not been electropolished, and four of AISI 304 that had been electropolished at different points in our system tank, so as to assess treatment uniformity.
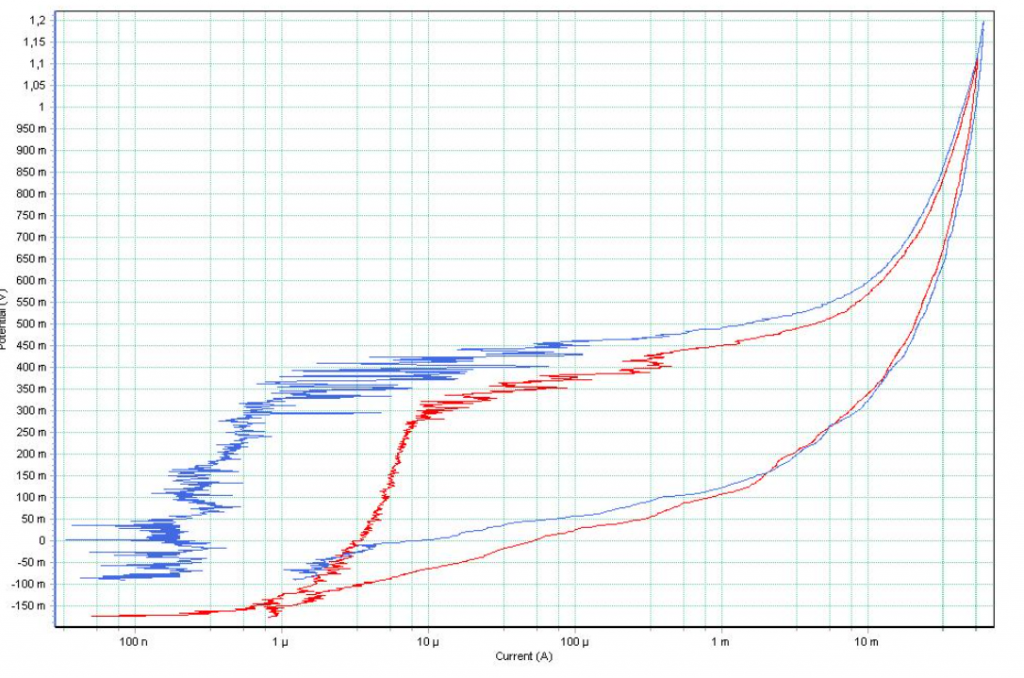
The progress of the polarisation curve showed that in all cases the electropolished sample presented a higher grade of passivation than the sample that had not been electropolished.
The graph compares the electropolished sample curve (blue) with that of the non-electropolished one (red). It can be seen that a higher level of passivation, as is the case of the electropolished sample, requires more electric current to trigger pitting.